Explain What Makes One Atom Different From Another Atom
What makes one element different from another is the number of protons in the nucleus of its atoms. What makes one atom of an element different from another.
The Structure Of The Atom Boundless Chemistry
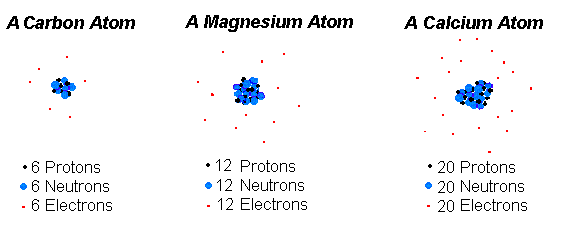
The number of protons in the nucleus which is generally equal to the number of orbiting electrons is what distinguishes atoms of one element from atoms of another element.
. The number of protons in an atom is called its atomic number. The defining particle that identifies an atom is the number of protons it contains. An example of atom and molecules would be water.
Answer 1 of 8. Examples of atoms include single particles of the elements of the periodic table such as sodium uranium argon and chlorine. Simultaneously the chlorine atom having gained an extra electron will take on a negative charge and become a.
9 Atoms of different elements with different mass numbers but same number of neutrons 10 Atoms of different elements having same atomic numbers DOWN 2 Atoms of same element having different mass number 3 Number of protons in an atoms 4 Proposed planetary model 6 Fundamental particle with no charge 7 Number of protons and neutrons. Well hydrogen is different from other elements in many ways. Its what makes one element different from another.
Most of an atom is empty space. The atomic number or z number or the number of protons is what makes one atom different from another. Many people might think atoms and elements are the same.
And the number of protons in the nucleus must change for one element to become another. The changing of one element into another called transmutation involves a change in the nucleus of the atom. One type of atom.
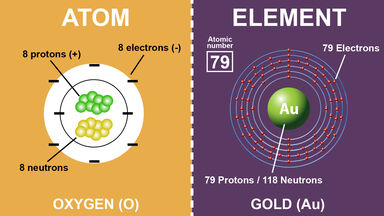
Protons and neutrons form the nucleus of an atom and a cloud of electrons orbits the nucleus. What makes atoms of one element different from the atoms of another element. An element is a substance that is made entirely from one type of atom.
If an atom were about as big as a baseball stadium the nucleus would be the size of a pea in the very center and the electrons would be somewhere on the outside edge. It is a molecule with a combination of two atoms of hydrogen and one atom of oxygen. The number of protons in an.
It therefore will have a positive charge and become a sodium ion. Atoms bond with one another so that they can lower their energy and become stable. Atoms are made up of three subatomic particles.
Although one could claim that these two words are strongly connected they are widely different. Lets use the model below to explain how atoms bond to become stable. Shared pairs of electrons fill the _____ energy levels of bonded atoms.
An ion is a charged atom or molecule. What makes an atom of gold different from an atom of iron is the number of protons neutrons and electrons inside it. So a particle that lacks protons is not an atom.
If it has more protons than electronsit is a positive ion. Typically these elements have the same number of negatively charged electrons orbiting. Firstlyit needs one election to complete its duplet.
Explore examples of elements and atoms. Ions usually form when electrons are transferred from one atom to another. An atom is the smallest component of an element containing neutrons protons and electrons and makes up everything around us.
However atoms and elements do have a few differences when you start breaking it down. Our current model of the atom can be broken down into three constituents parts protons neutron and electrons. What makes each element different is the number of positively charged protons in the nucleus of the atom.
A large difference between electronegativity values between atoms indicates one atom is attracted to electrons while the other can accept electrons. If the atom has more electrons than protons it is a negative ion or ANION. Learn other differences between atoms and elements by dissecting these two terms.
Correct me if Im wrong. The main difference is elements are made of atoms. The number of neutrons can vary to produce isotopes which are atoms of the same element that have different numbers of neutrons.
Simultaneouslyit can also lose one electron to form a cation. An element is a substance in which all of the atoms have the same atomic proton number. However even one lone proton is an atom of hydrogen.
Explain How Elements And Atoms Are RelatedA particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons. One of the primary differences between molecules and atoms is that the former latter cannot be divided further like the former. Normally the number of electrons is equal to the number of protons which makes atoms electrically neutral.
The number of protons in an atom is the defining feature of an atom. And as atoms bond with other atoms they often make molecules with unique chemical and physical properties. The number of protons determines an elements atomic number Z and distinguishes one element from another.
This type of bond forms between a metal atom and a. Due to this anomaly its place in periodic table is controversial. When an atom is attracted to another atom because it has an unequal number of electrons and protons the atom is called an ION.
When the negatively charged atom anion and the positively charged atom cation. Structure Of The Atom. For example the element hydrogen is made from atoms conta.
An atom that has more or fewer electrons in orbit than protons in its nucleus is called an ionOnce the electron from its valence shell has been transferred the sodium atom will be missing an electron. These atoms usually form ionic bonds with each other. An atom is the smallest particle.
For example carbons atomic number Z is 6 because it has 6 protons.

No comments for "Explain What Makes One Atom Different From Another Atom"
Post a Comment